Farheen Khan, (Hons)B.Sc. (Biochemistry) [Get Well Clinic]
The year 2020 will forever be remembered as the year of COVID-19, social distancing, cancelled plans, and more. Luckily, it is coming to an end (finally) with promising results from Pfizer/BioNTech and Moderna regarding the availability of COVID-19 vaccines by early 2021. These vaccines are one type of many different vaccine systems being developed and produced for protection against COVID19.
Both vaccines are messenger RNA (mRNA) vaccines.1,2 Before going on to further explain how these vaccines work, I’d like to first assure that the mRNA engineered for the Pfizer and Moderna vaccines will not integrate into and remain in our DNA forever. Here’s why:
If we look at the central dogma process, our DNA (genetic material) is transcribed into RNA in the nucleus of our cells.3 This RNA is then processed to become mature mRNA. After, this mRNA is transported to the cytoplasm of our cells (thus called messenger RNA), where it is decoded and translated into corresponding proteins.3
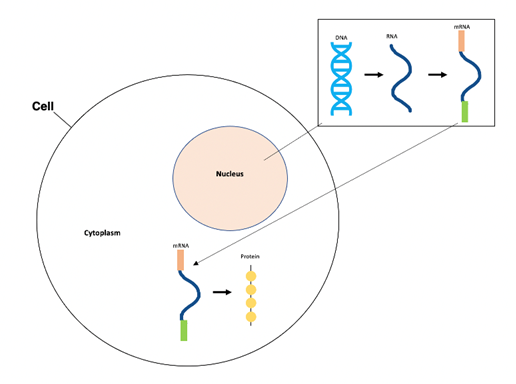
Figure 1. The central dogma process includes DNA transcription into RNA (in nucleus), RNA processing to mRNA (in nucleus), and mRNA translation to protein product (in cytoplasm).
When someone is injected with the mRNA in the Pfizer or Moderna vaccines, the mRNA travels encapsulated in lipid nanoparticles to the cytoplasm of the individual’s cells.4,5 The mRNA then serves as genetic instructions for the cells and is translated to create the engineered protein of interest. In this case, the protein of interest is a version or part of the SARS-CoV-2 spike protein.4,5
Now, you may be wondering, “why the spike protein?” This is because it is the spike protein of the virus that interacts with a specific receptor, the ACE-2 receptor, on the surface of our cells.6 Upon interaction, the virus is internalized into our cells, and proceeds to take over our cells’ machinery, repurposing them to create more copies of the virus. These infected cells then release millions of copies of the virus which then go on to infect other cells in our bodies, ultimately causing the COVID-19 disease.7
The presence of this version or fragment of the spike protein then elicits an immune response as the individual’s body recognizes the protein as a foreign substance.8 More specifically, the immune system produces antibodies against the protein and executes other necessary immunogenic responses as well.5,9 Since only one protein of the virus would be created, the vaccine would not infect a vaccinated individual with COVID-19.10
As a note, antibodies are large Y-shaped proteins that stick to the surface or part of a virus or bacteria, tagging it for attack by other elements of the immune system to ultimately neutralize the virus or bacteria.11 Antibodies are designed to bind to and attack one kind of virus or bacteria only.11 This means that an antibody that is for instance designed to destroy the common cold virus, cannot be used to attack the SARS-CoV-2 or Influenza A viruses.
As antibodies specific to the SARS-CoV-2 spike protein are produced upon administration of the Pfizer or Moderna vaccine, if the SARS-CoV-2 virus does enter the vaccinated individual’s body at a later time, the antibodies produced post-vaccination will be able to recognize the spike proteins of the virus and thus bind to them.12 As a result, the virus will be unable to bind to the ACE-2 receptors on the surface of the individual’s cells, and therefore will not be internalized, rendering it incapable of taking over the cells’ machinery and replicating.12 In addition, the binding of the antibodies to the spike proteins present on the surface of the SARS-CoV-2 virus will also tag the virus for degradation by other elements of the immune system.12
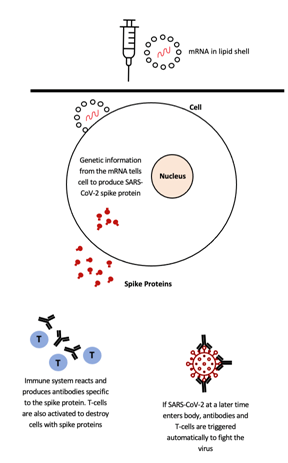
Figure 2. The engineered mRNA is injected into an individual’s body in a lipid nanoparticle. The mRNA is then translated into a fragment or version of the SARS-CoV-2 spike protein in the cytoplasm of the individual’s cells. As the spikes are released from the individual’s cells, their immune system produces antibodies and releases T-cells to execute necessary immunogenic reactions. This image has been adapted from a Figure in an article in The Washington Post: “What you need to know about the AstraZeneca, Moderna and Pfizer vaccines”.12
While the injected mRNA remains in vaccinated individuals’ bodies just long enough to be translated, the resulting protein stick around long enough to induce the production of antibodies. After, both the mRNA and protein are degraded.8
Now that we know how the mRNA vaccines work, it is important to note that this is new technology. Before 2020, mRNA vaccines have never been authorized to be used for humans due to their unknown short- and long-term effects.8
Further, due to the fragility of mRNA, mRNA vaccines must be kept at very low temperatures to prevent degradation.13 The Pfizer vaccine needs to be stored at -70°C during transportation, at which it will last for up to 10 days.14 In ultra-low-temperature freezers, the vaccine’s shelf life extends to six months, but in refrigeration units commonly available in hospitals (2°C-8°C), the Pfizer vaccine can only last for up to five days.14 On the other hand, the Moderna vaccine can last up to six months at -20°C (equivalent to a regular freezer), and in refrigeration units (2°C-8°C), the Moderna vaccine remains stable for 30 days.15
In addition, both vaccines require two doses to be administered.2,16 For the Pfizer vaccine, patients would receive their second dose 21 days after the first dose (30μg each), whereas for the Moderna vaccine, patients would receive their second dose 28 days after the first dose (an additional week later; 100μg each).2,16
Table 1. Similarities and Differences Between Two COVID-19 mRNA Vaccines
|
Pfizer Vaccine |
Moderna Vaccine |
|
|
Type of Vaccine |
mRNA |
mRNA |
|
Shelf-life at Normal Refrigeration Temperatures (2°C-8°C) |
5 days |
30 days |
|
mRNA Dosage Required |
Two 30μg doses, 21 days apart |
Two 100μg doses, 28 days apart |
|
# of Phase 3 Trial Participants |
> 43,000 |
> 30,000 |
|
Participants’ Age and Health Conditions |
Age groups: 12-15 (100 participants only); 18-55; 65-85 Health Conditions: Healthy individuals with and without prior SARS-CoV-2 infection; Individuals with stable pre-existing disease; Individuals with stable, chronic HIV, HCV, and HBV infection |
Age: 18+ Health Conditions: Healthy with no previous history of SARS-CoV-2 infection; Individuals with stable pre-existing medical conditions |
|
Trial Design |
Randomized, blinded, placebo-controlled |
Randomized, blinded, 1:1 placebo-controlled |
|
Placebo Used |
Saline solution |
Saline solution |
|
Efficacy |
95% (>94% in individuals over the age of 65) |
94.1% |
|
When was Vaccine Efficacy Measured? |
7 days after second dose (day 28) |
14 days after second dose (day 42) |
|
Observed Side Effects* |
Fatigue, Headache |
Fatigue, Muscle Aches, Stiff Joints, Headache, Pain, Redness at Injection Site |
|
How Long Will Participants Be Monitored? |
2 years after second dose |
2 years after second dose |
*For more details on the observed frequency and severity of the side effects, please read the section titled “Additional Information Regarding Results Observed in the Phase 3 Clinical Trials” below.
It is important to note that peer-reviewed articles with the data above have not yet been published but will be soon. Most of the up-to-date information at the moment can only be retrieved from Pfizer and Moderna’s press releases.
Finally, though the vaccines do sound promising, there still remains a lot to learn:
- Although the vaccines seem effective at the moment, how effective will they be in the long term? By continuously monitoring trial participants, Moderna and Pfizer will get a better idea of how long the immunity resulting from the vaccines will last. Will the vaccine provide immunity for decades like the measles vaccine, or will people need to get frequent boosters?17
- Both vaccines prevent the COVID-19 disease effectively. However, it is unknown if both vaccines also prevent infection (replication of the virus). If they do, transmission and asymptomatic spread can be blocked as well.18
- The safety profile for both vaccines is promising and generally tolerable according to the most recent data. Trial participants still need to be monitored for months and years to determine long-term side effects.17 Though we do not know the long-term side effects of the vaccine just yet, we do know that COVID-19 itself can have long-term effects including fatigue and lung damage.19
It has definitely been one long year, but the fact that we are ending 2020 with two promising and effective vaccine candidates is no less than exciting. Both the Pfizer and Moderna vaccines have surpassed the minimum threshold set by the Food and Drug Administration (FDA) for consideration of an emergency approval.2,20 Nonetheless, in the meantime, it is important for all of us to continue to stay educated, socially distanced (even when the vaccines do come out as not everyone will be able to get immunized at once), healthy, and safe until the vaccines are ready for distribution and administration.
ADDITIONAL INFORMATION REGARDING RESULTS OBSERVED IN THE PHASE 3 CLINICAL TRIALS
Pfizer executed their randomized, blinded, placebo-controlled Phase 3 trials with over 43,000 participants.1,21 Pfizer then measured the efficacy of the vaccine 28 days after trial participants received their first dose (seven days after receiving their second dose).21 The vaccine was found to be 95% effective as from the 170 positive confirmed cases that were evaluated, 162 were observed in the placebo group.1 This was measured in participants over the age of 18 both with and without prior SARS-CoV-2 infection.1 Pfizer has also submitted solicited safety data on approximately 100 children, aged 12-15 in their request to the FDA for Emergency Use Authorization.20 As Pfizer mentioned in their press release on Wednesday November 18th, 2020, “this efficacy was consistent across age, gender, race and ethnicity demographics; observed efficacy in adults over 65 years of age was over 94% ... There were 10 severe cases of COVID-19 observed in the trial, with nine of the cases occurring in the placebo group … To date, the Data Monitoring Committee for the study has not reported any serious safety concerns related to the vaccine … The only Grade 3 (severe) solicited adverse events greater than or equal to 2% in frequency after the first or second dose were fatigue at 3.8% and headache at 2.0% following dose 2. Consistent with earlier shared results, older adults tended to report fewer and milder solicited adverse events following vaccination.”1 Trial participants have been followed for at least two months after receiving the second dose. Pfizer plans to monitor participants’ health for two years after receiving the second dosage of the vaccine candidate or placebo.20
More information about the Pfizer vaccine and its Phase 3 trial can be found at:
- https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- https://www.pfizer.com/science/coronavirus
Moderna executed their randomized, blinded, 1:1 placebo-controlled study Phase 3 trials with more than 30,000 participants.2,22 Moderna then measured the efficacy of the vaccine 42 days after trial participants received their first dose (14 days after receiving their second dose).2 The vaccine was found to be 94.1% effective; from the 196 positive confirmed cases that were evaluated, 185 were observed in the placebo group.2 30 severe cases were confirmed, of which all occurred in the placebo group as well.2 One COVID-19-related death was reported, however, this too occurred in the placebo group.2 According to Moderna’s press release from November 30th, 2020, “efficacy was consistent across age, race and ethnicity and gender demographics.”2 This was measured in healthy participants over the age of 18 who either had no previous history of COVID-19 or had stable pre-existing medical conditions at time of screening, putting them at higher risk of severe COVID-19.23 Moderna’s Phase 3 trial also assessed approximately 7000 participants over the age of 65 and observed similar “binding- and neutralizing-antibody responses similar to those observed and reported among vaccine recipients between the age of 18 and 55 and above the “median of a panel of controls who had donated convalescent serum” (serum from individuals who had recently recovered from COVID-19).24 According to Moderna’s press release from November 16, 2020, the vaccine “did not report any significant safety concerns … The majority of adverse events were mild or moderate in severity. Grade 3 (severe) events greater than or equal to 2% in frequency after the first dose included injection site pain (2.7%), and after the second dose included fatigue (9.7%), myalgia [muscle aches] (8.9%), arthralgia [joint stiffness] (5.2%), headache (4.5%), pain (4.1%), and erythema/redness at the infection site (2.0%). These solicited adverse events were generally short-lived.”25 According to Moderna’s Phase 3 study overview, “trial participants will be asked to return to the study site three more times over approximately a year. Participants will have one final visit to the study site approximately two years from the date of their second injection.”23
More information about the Moderna vaccine and its Phase 3 trial can be found at:
- https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study
- https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
- https://www.modernatx.com/cove-study
REFERENCES:
(1) Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints | Pfizer https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed Dec 2, 2020).
(2) Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization | Moderna, Inc. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study/ (accessed Dec 2, 2020).
(3) The Central Dogma | Protocol https://www.jove.com/science-education/10798/the-central-dogma (accessed Dec 2, 2020).
(4) Mulligan, M. J.; Lyke, K. E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K. A.; Li, P.; Koury, K.; Kalina, W.; Cooper, D.; Fontes-Garfias, C.; Shi, P.-Y.; Türeci, Ö.; Tompkins, K. R.; Walsh, E. E.; Frenck, R.; Falsey, A. R.; Dormitzer, P. R.; Gruber, W. C.; Şahin, U.; Jansen, K. U. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586 (7830), 589–593. https://doi.org/10.1038/s41586-020-2639-4.
(5) Jackson, L. A.; Anderson, E. J.; Rouphael, N. G.; Roberts, P. C.; Makhene, M.; Coler, R. N.; McCullough, M. P.; Chappell, J. D.; Denison, M. R.; Stevens, L. J.; Pruijssers, A. J.; McDermott, A.; Flach, B.; Doria-Rose, N. A.; Corbett, K. S.; Morabito, K. M.; O’Dell, S.; Schmidt, S. D.; Swanson, P. A.; Padilla, M.; Mascola, J. R.; Neuzil, K. M.; Bennett, H.; Sun, W.; Peters, E.; Makowski, M.; Albert, J.; Cross, K.; Buchanan, W.; Pikaart-Tautges, R.; Ledgerwood, J. E.; Graham, B. S.; Beigel, J. H. An MRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020, 383 (20), 1920–1931. https://doi.org/10.1056/NEJMoa2022483.
(6) Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367 (6485), 1444–1448. https://doi.org/10.1126/science.abb2762.
(7) Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, Transmission, and Pathogenesis of SARS-CoV-2. BMJ 2020, 371. https://doi.org/10.1136/bmj.m3862.
(8) CDC. Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html (accessed Dec 2, 2020).
(9) Walsh, E. E.; Frenck, R. W.; Falsey, A. R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M. J.; Bailey, R.; Swanson, K. A.; Li, P.; Koury, K.; Kalina, W.; Cooper, D.; Fontes-Garfias, C.; Shi, P.-Y.; Türeci, Ö.; Tompkins, K. R.; Lyke, K. E.; Raabe, V.; Dormitzer, P. R.; Jansen, K. U.; Şahin, U.; Gruber, W. C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 0 (0), null. https://doi.org/10.1056/NEJMoa2027906.
(10) Abbasi, J. COVID-19 and MRNA Vaccines—First Large Test for a New Approach. JAMA 2020, 324 (12), 1125. https://doi.org/10.1001/jama.2020.16866.
(11) July 17, T. G.-A. M. E.; 2020. What are antibodies? https://www.livescience.com/antibodies.html (accessed Dec 2, 2020).
(12) What you need to know about the AstraZeneca, Moderna and Pfizer vaccines https://www.washingtonpost.com/health/2020/11/17/covid-vaccines-what-you-need-to-know/ (accessed Dec 2, 2020).
(13) Why Does Pfizer’s COVID-19 Vaccine Need To Be Kept Colder Than Antarctica? https://www.npr.org/sections/health-shots/2020/11/17/935563377/why-does-pfizers-covid-19-vaccine-need-to-be-kept-colder-than-antarctica (accessed Dec 2, 2020).
(14) COVID-19 Vaccine U.S. Distribution Fact Sheet | Pfizer https://www.pfizer.com/news/hot-topics/covid_19_vaccine_u_s_distribution_fact_sheet (accessed Dec 2, 2020).
(15) Moderna Announces Longer Shelf Life for its COVID-19 Vaccine Candidate at Refrigerated Temperatures | Moderna, Inc. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine/ (accessed Dec 2, 2020).
(16) Pfizer and BioNTech Choose Lead mRNA Vaccine Candidate Against COVID-19 and Commence Pivotal Phase 2/3 Global Study | Pfizer https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-choose-lead-mrna-vaccine-candidate-0 (accessed Dec 2, 2020).
(17) What We Know and What We Don’t About the Moderna and Pfizer COVID-19 Vaccines https://www.kqed.org/science/1971077/what-we-know-and-what-we-dont-about-the-moderna-and-pfizer-covid-19-vaccines (accessed Dec 2, 2020).
(18) MedCram - Medical Lectures Explained CLEARLY. Coronavirus Update 117: Moderna vs. Pfizer COVID 19 Vaccine (MRNA Vaccines); 2020.
(19) Marshall, M. The Lasting Misery of Coronavirus Long-Haulers. Nature 2020, 585 (7825), 339–341. https://doi.org/10.1038/d41586-020-02598-6.
(20) Pfizer and BioNTech to Submit Emergency Use Authorization Request Today to the U.S. FDA for COVID-19 Vaccine | Pfizer https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-submit-emergency-use-authorization (accessed Dec 2, 2020).
(21) Coronavirus COVID-19 Scientific Research and Resources | Pfizer https://www.pfizer.com/science/coronavirus (accessed Dec 3, 2020).
(22) MRNA-1273-P301-Protocol.Pdf.
(23) Moderna’s Fully Enrolled Phase 3 COVE Study of mRNA-1273 | Moderna, Inc. https://www.modernatx.com/cove-study (accessed Dec 2, 2020).
(24) Anderson, E. J.; Rouphael, N. G.; Widge, A. T.; Jackson, L. A.; Roberts, P. C.; Makhene, M.; Chappell, J. D.; Denison, M. R.; Stevens, L. J.; Pruijssers, A. J.; McDermott, A. B.; Flach, B.; Lin, B. C.; Doria-Rose, N. A.; O’Dell, S.; Schmidt, S. D.; Corbett, K. S.; Swanson, P. A.; Padilla, M.; Neuzil, K. M.; Bennett, H.; Leav, B.; Makowski, M.; Albert, J.; Cross, K.; Edara, V. V.; Floyd, K.; Suthar, M. S.; Martinez, D. R.; Baric, R.; Buchanan, W.; Luke, C. J.; Phadke, V. K.; Rostad, C. A.; Ledgerwood, J. E.; Graham, B. S.; Beigel, J. H. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 0 (0), null. https://doi.org/10.1056/NEJMoa2028436.
(25) Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study | Moderna, Inc. https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy/ (accessed Dec 2, 2020)